Frequently Asked Questions
What is antibiotic resistance?
What causes antibiotic resistance?
Who is responsible for the development of antibiotic resistance?
Why is antibiotic resistance a One-Health issue?
Why should we care about antibiotic resistance?
Is antibiotic resistance a real threat?
What is being done to fight antibiotic resistance?
Why don’t we create new antibiotics to fight resistance?
What’s the difference between an antibiotic, ARB, and ARG?
What are antibiotic resistance genes (ARGs) and why are they of concern?
What happens to antibiotics after they are given to a person or an animal?
What are alternative options for addressing bacterial infections?
What is the Veterinary Feed Directive?
How can antibiotics given to livestock affect our produce?
What is antibiotic resistance?
Antibiotic resistance is the ability of some bacteria to survive antibiotic treatments that are meant to kill them or stop their growth. Bacteria can be naturally resistant to certain antibiotics. For example, an antibiotic that specifically targets Gram-positive bacteria will not affect Gram-negative bacteria. Antibiotic drugs are usually created with these properties of certain bacteria in mind. However, bacteria can also acquire resistance, either through mutation or through sharing of antibiotic resistance genes (ARG). Once acquired, the resistance will be passed on to future bacteria when the resistant bacteria replicate.

What causes antibiotic resistance?
Antibiotic resistance is actually a natural phenomenon- we have just greatly sped up the process by misuse and overuse in both human and animal medicine. The more antibiotics are used, the greater the chance that selection pressure will contribute to the spread of resistance. Inappropriate antibiotic use for disease that is not bacterial in origin (such as viral disease) and not taking appropriate doses of antibiotics for the full course of treatment have also contributed to resistance.
Who is responsible for the development of antibiotic resistance?
While much attention is placed on the agricultural industry for worsening antibiotic resistance, the reality is that any antibiotic use, whether it is in humans or animals, contributes to this public health dilemma. Too much time has been spent pointing fingers at various industries and it is imperative that we all take responsibility for the stewardship of these life-saving drugs in order to preserve their efficacy.
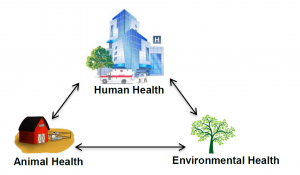
Why is antibiotic resistance a One-Health issue?
One-Health is an initiative that highlights the interdependence of human, animal, and environmental health. It encourages a trans-disciplinary approach at national and global levels to fight public health problems and achieve the best health possible for humans, animals, and the environment.
Antibiotic resistance represents a quintessential One-Health issue because investigating and solving this problem requires the efforts of many disciplines, such as engineers, veterinarians, doctors, and farmers, to just name a few. Antibiotic resistance is happening at the global level and working across boarders is also an important aspect of combating it.
For more information on One-Health, visit: https://www.usda.gov/topics/animals/one-health
Why should we care about antibiotic resistance?
Antibiotic resistance is a global challenge for human and animal health. The more antibiotic resistance spreads, the less effective antibiotics become for fighting disease. If antibiotics lose efficacy, people may start suffering from simple bacterial infections and those requiring procedures that compromise the immune system may be more prone to serious bacterial infections. Chemotherapy and major surgeries will become much more dangerous processes than they already are. From an animal health standpoint, antibiotics greatly enhance animal welfare and provide large economic benefits in the agricultural industry by preventing disease and enhancing growth.
Is antibiotic resistance a real threat?
The incidence of antibiotic resistant infections in people has risen dramatically in recent years. The Centers for Disease Control and Prevention (CDC) estimate that at least 2,049,442 illnesses and 23,000 deaths in the U.S. alone can be attributed to antibiotic resistance. These numbers indicate that antibiotic resistance is a public health challenge and will continue to be if action is not taken.
One particular challenge we face is the lack of data (as of 2017) that tells us what amount of exposure to antibiotics, antibiotic resistant bacteria (ARB), or antibiotic resistance genes (ARG) pose a human health risk. Currently, the implication is that higher levels of antibiotics, ARBs, or ARGs indicate a higher level of risk of spreading antibiotic resistance and more individuals contracting resistant infections. However, having published guidelines that tell us how many antibiotics, ARBs, or ARGs is a real risk will be an invaluable tool in fighting antibiotic resistance and mitigating exposure to practices that can potentially spread antibiotic resistance amongst people and animals.
What is being done to fight antibiotic resistance?
Scientists from all over the world in many different fields are researching the causes and outcomes of antibiotic resistance. Additionally, new laws such as the Veterinary Feed Directive (VFD) are in effect in order to increase judicious antibiotic use. In human medicine, initiatives such as “Get Smart,” piloted by the Centers for Disease Control and Prevention (CDC) are educating and encouraging doctors to definitively diagnose patients to ensure antibiotics are only prescribed for bacterial infections. Public awareness has also played an important role in communicating the importance of judicious antibiotic use. For example, there is greater awareness of the importance of finishing an entire antibiotic course of treatment by taking all of the antibiotics prescribed by the doctor.
CDC Get Smart about Antibiotics Initiative: https://www.cdc.gov/getsmart/
Why don’t we create new antibiotics to fight resistance?
The development of new antibiotic drugs to replace those antibiotics that have lost their efficacy is an obvious solution to the antibiotic resistance problem. However, the development of antibiotics has drastically slowed in recent decades. Antibiotic discovery is an expensive and technically difficult process and has become even more difficult now that many of the easier-to-develop drugs have already been discovered. According to the World Health Organization (WHO), there are also few drug companies investing in antibiotic development since they traditionally “have a poor return on investment because they are taken for a sspes period of time and cure their target disease.”
This timeline shows the lack of new antibiotic class discoveries in the last several decades compared to the majority of the 20th century.

WHO Reference: Bulletin of the World Health Organization 2011;89:88–89. doi:10.2471/BLT.11.030211
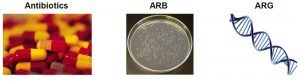
What’s the difference between an antibiotic, ARB, and ARG?
ARGs are a part of antibiotic resistant bacteria (ARB) DNA that encodes the ability for bacteria to live in the presence of antibacterial drugs. ARGs are of particular concern because they can transfer between bacteria, via horizontal gene transfer, which is when genes as movable, independent pieces are inserted into another bacterium’s genome. In other words, bacteria that are not resistant can receive an ARG from other bacteria and become resistant.
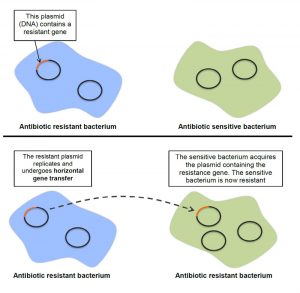
What are antibiotic resistance genes (ARGs) and why are they of concern?
ARGs are a part of antibiotic resistant bacteria (ARB) DNA that encodes the ability for bacteria to live in the presence of antibacterial drugs. ARGs are of particular concern because they can transfer between bacteria, via horizontal gene transfer, which is when genes as movable, independent pieces are inserted into another bacterium’s genome. In other words, bacteria that are not resistant can receive an ARG from other bacteria and become resistant.
What is metagenomics?
Metagenomics is a method researchers use to search bacterial DNA for antibiotic resistance genes (ARGs). It involves directly isolating bacterial DNA from manure, soil, water, and other environmental samples and then comparing the DNA to databases used by researchers all around the world in order to determine what ARGs may be present. As of now, there are hundreds to thousands of known ARGs, with attention to any new ARGs that could enable pathogens to be resistant of particular interest.
What happens to antibiotics after they are given to a person or an animal?
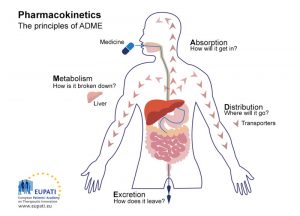
When antibiotics are given, they go through several different levels of interaction in the body. These processes are the same for both humans and animals.
Absorption: The first process an antibiotic must go through in the body is absorption into the bloodstream, regardless if it is given via injection or orally. Antibiotics are absorbed into the bloodstream at different rates, depending on the drug and route of administration. Additionally, many drugs are not 100% absorbed and will go directly to the excretion process.
Distribution: Blood acts as a “highway” for the antibiotic after it is absorbed. Through the process of distribution, the antibiotic uses the blood to travel throughout the body to different tissues. The antibiotic’s molecular properties will determine where it will travel and accumulate in the body.
Metabolism: Antibiotic metabolism is the process of breaking down the drug into its metabolites, which are smaller and less therapeutic versions of the antibiotic. The body does this in preparation to eliminate the antibiotics from the body. Common organs that metabolize drugs include the liver and kidneys.
Excretion: The metabolites of the antibiotic as well as drug that was never absorbed and used are removed from the body, usually via the urine and feces.
Understanding what happens to antibiotics after they are given to people and animals, especially with regard to excretion, can help us answer questions about antibiotic resistance. Knowing how much antibiotic is excreted from the body can help us develop practices that reduce the risk of antibiotics in or on our food as well as decrease antibiotic resistant bacteria and/or antibiotic resistance genes.
For further information, visit: http://pharmaxchange.info/press/2011/04/pharmacokinetics-basics-absorption-distribution-metabolism-and-excretion/
What are alternative options for addressing bacterial infections?
While antibiotics are used to treat bacterial infection, they are not the only tools available for use. The best solution to bacterial infections, both economically and for the health of the person or animal, is prevention. Prevention is not always easy and requires involved management practices such as hygiene, vaccination, biosecurity, nutrition, and immune support. However, the long-term benefits outweigh the sspes-term costs. Many of the same practices for prevention may also be used as alternative options for addressing bacterial infections.
How can we reduce transmission of antibiotics and antibiotic resistant bacteria (ARB) from farm to fork?
Antibiotics themselves will generally diminish in concentration along each step from farm to fork. ARBs, on the other hand, are living organisms and may die off or grow to higher concentrations depending on whether the environment is suitable. Currently, antibiotics are monitored to ensure there is little to no residue in our milk and meat products. Veterinarians and producers have access to databases such as the Food Animal Residue Avoidance Databank (FARAD), which gives detailed information regarding withdrawal times for numerous drugs.
Unlike antibiotics, there are no specific guidelines for monitoring ARBs. However, there are barriers in place to prevent the spread of harmful bacteria (pathogens), such as the Food Safety Modernization Act (FSMA), which may help limit the spread of antibiotic resistance. FSMA contains specific information regarding techniques aimed at killing or reducing the number of pathogens in food. Some examples of these techniques include composting, heat treatments, high pressure processing (HPP), irradiation, refrigeration, and freezing. The U.S. Environmental Protection Agency (EPA) likewise has strict regulations regarding manure runoff into water supply sources as well as soil amendments originating from manure. The National Pollutant Discharge Elimination System (NPDES) permit in particular is required for livestock and poultry production, which helps prevent pathogen contamination of drinking water and crops.
What is the Veterinary Feed Directive?
The Veterinary Feed Directive (VFD) is an initiative of the U.S. Food and Drug Administration (FDA) that became effective January 1, 2017. The VFD requires authorization from a veterinarian in order to use medically important antibiotics in animal feed or water. Medically important drugs are antibiotics that are used in both humans and animals, but are particularly important for human medicine. The VFD is intended to help promote the responsible use of medically important antibiotics in food-producing animals by stopping the use of antibiotics for growth promotion or feed efficiency purposes, while still allowing their use to control and treat certain diseases under the expertise of a veterinarian.
For more information about the VFD visit:
VFD Central: http://www.feedstuffs.com/vfd-central
Cornell University College of Veterinary Medicine VFD Information: https://ahdc.vet.cornell.edu/programs/NYSCHAP/nysvfrp/vfd.cfm
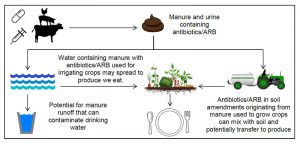
How can antibiotics given to livestock affect our produce?
Nearly all of the antibiotics administered to livestock will end up in their urine and manure as the original antibiotic or metabolites. Without effective sanitation and protective barriers, manure and urine containing antibiotics and antibiotic resistant bacteria (ARB) can contaminate drinking water as well as soil and water used to grow produce. Since manure-based amendments, like compost, are typically used to fertilize crops, manure-derived antibiotics/ARB have the potential to spread to the soil and crops.
What is a microbiome?
A microbiome is a collection of microorganisms (bacteria, viruses, fungi, protists, archaea) found in or on an organism. These microorganisms may be good or bad (pathogenic). Microbiomes differ between and within species, meaning one animal’s microbiome is different from another’s. Humans, animals, and plants all have a microbiome.
The microbiome may be important in combating the spread of antibiotic resistance from farm to fork since the vegetables we eat have a microbiome. Ensuring this microbiome is free of antibiotic resistant bacteria (ARB) and/or antibiotic resistance genes (ARG) is important so that they do not spread to humans. Soil amendments such as manure as well as sanitation practices are important factors in determining the safety of the vegetable microbiome.
More information on the plant microbiome: https://www.sciencedaily.com/releases/2016/07/160712130223.htm
What is composting?
Composting is the decomposition of organic material that turns into a rich soil called compost, which can be used as a soil amendment to enhance the growth of crops. Composting can be done everywhere- from large farms to your own backyard. While all organic material will naturally decay by itself, composting can improve soil quality and reduce solid waste.
Composting decreases both ammonium and soluble nitrate, which increases soil fertility. Additionally, the environmental conditions that composting requires, such as very high temperatures and oxygen access for a prolonged period of time, make an unfavorable place for pathogens to live. By killing possibly harmful bacteria, composting may improve public health by decreasing exposure to potentially dangerous pathogens, including antibiotic resistant bacteria (ARB).
More information on composting: http://extension.psu.edu/business/start-farming/soils-and-soil-management/compost-how-to-make-it-and-how-much-to-use
